PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) (“PureTech” or the “Company”), a clinical-stage biotherapeutics company, is pleased to note that its Founded Entity, Sonde Health (“Sonde”), today announced that it will collaborate with leading chipmaker Qualcomm Technologies, Inc. (“Qualcomm”) to optimize use of Sonde’s vocal biomarker technology on the flagship and high-tier Qualcomm® Snapdragon™ 888 and 778G 5G Mobile Platforms to help bring native, machine learning-driven vocal biomarker capabilities to mobile and IoT devices globally. The optimization has the potential to unlock several native health screening and monitoring applications on up to the hundreds of millions of mobile devices that use these Snapdragon mobile platforms.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210708005107/en/
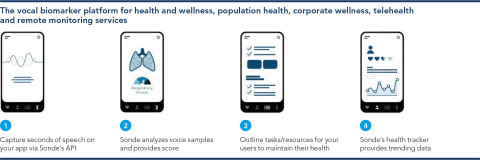
PureTech’s Founded Entity, Sonde Health, today announced that it will collaborate with leading chipmaker Qualcomm for use of Sonde’s vocal biomarker technology on the flagship and high-tier Qualcomm Snapdragon 888 and 778G 5G Mobile Platforms to help bring native, machine learning-driven vocal biomarker capabilities to mobile and IoT devices globally. (Graphic: Business Wire)
The full text of the announcement from Sonde is as follows:
Sonde Health Vocal Biomarker Technology Optimized on Qualcomm Snapdragon Mobile Platforms
BOSTON — July, 8 2021 — Sonde Health announced that it will work with leading chipmaker Qualcomm Technologies, Inc. to optimize Sonde’s vocal biomarker technology for use with the flagship and high-tier Qualcomm® Snapdragon™ 888 and 778G 5G Mobile Platforms to help bring native, machine learning-driven vocal biomarker capabilities to mobile and IoT devices globally. The optimization has the potential to unlock several native health screening and monitoring applications on hundreds of millions of mobile devices that use these Snapdragon mobile platforms.
“Imagine a car that can detect whether a driver is too impaired to drive safely, or a home hub that can detect the onset of depression, or a phone that can make continuous asthma assessments. Bringing the vocal biomarker technology directly into mobile hardware will make new health features more useful and secure,” said David Liu, CEO at Sonde Health. “This collaboration marks a tremendous boost to our growth strategy, a vote of confidence in our technology, and a giant leap forward for preventive and personalized health care.”
Sonde will work with Qualcomm Technologies to introduce Sonde’s capabilities and technology concept to Qualcomm Technologies’ mobile and IoT customers as part of the collaboration.
Sonde Health is a leading innovator in the field of vocal biomarker technology, which uses audio signal processing and machine learning to identify changes in the human voice that may be indicative of a health condition. With a 6-second voice sample, Sonde’s product, Sonde One, can detect symptoms of asthma, COPD, and other respiratory illnesses, and can be used as an early warning system for COVID-19. Sonde Health’s self-serve API/Software Development Kit lets developers quickly integrate the company’s voice-enabled system check into Android and iOS applications.
With Sonde’s technology optimized for use with Snapdragon 888 and 778G, device makers can enable vocal biomarker monitoring as a device feature for their users. And because the capability is native to the device, users simply opt-in to allow voice processing to occur in the phone. This eliminates the need to send health data to the cloud. The result is faster, more secure results with a reduced adherence burden on patients.
“Patients with chronic conditions like asthma aren’t going to have to painstakingly log in their daily respiratory diaries. They’ll be able to continuously monitor their health without a watch, ring, or other wearable device, simply by doing what they normally do on their phone,” Liu said.
For OEMs (Original Equipment Manufacturers) in the mobile device and IoT industries, native vocal biomarker capabilities can provide a crucial differentiator that helps protect the health and safety of consumers.
“We are excited to talk to OEMs to explore new ways to deploy innovative health monitoring capabilities that users will soon come to expect from their web-enabled products,” Liu said.
About Sonde Health
Leveraging over 1 million voice samples from 80,000+ individuals, Sonde Health’s proprietary voice-based technology platform is designed to detect changes of health conditions – like mental fitness and respiratory disease – from changes in voice. Using advanced audio signal processing and machine learning, Sonde senses and analyzes subtle vocal changes due to changes in a person’s physiology to provide early health detection and monitoring.
Sonde One, its health screening app, helps large organizations to execute a daily population screening regimen that can help reduce the spread of COVID-19, comply with government mandates, and return to work safely. Sonde also has broad intellectual property coverage worldwide and licenses its technology through its API platform. www.sondehealth.com
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to discovering, developing and commercializing highly differentiated medicines for devastating diseases, including inflammatory, fibrotic and immunological conditions, intractable cancers, lymphatic and gastrointestinal diseases and neurological and neuropsychological disorders, among others. The Company has created a broad and deep pipeline through the expertise of its experienced research and development team and its extensive network of scientists, clinicians and industry leaders. This pipeline, which is being advanced both internally and through PureTech's Founded Entities, is comprised of 26 therapeutics and therapeutic candidates, including two that have received FDA clearance and European marketing authorization, as of the date of PureTech’s most recently filed Annual Report on Form 20-F. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points based on the Company's unique insights into the biology of the brain, immune and gut, or BIG, systems and the interface between those systems, referred to as the BIG Axis.
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, expectations regarding the collaboration between Sonde and Qualcomm, including the expected optimization of Sonde’s vocal biomarker technology, expectations regarding the potential of Sonde’s vocal biomarker technology, including use on the Snapdragon mobile platforms, expectations regarding the validation of Sonde’s technology, expectations regarding the potential benefits to patients from the collaboration and those risks and uncertainties described in the risk factors included in the regulatory filings for PureTech Health plc. These forward-looking statements are based on assumptions regarding the present and future business strategies of the company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, neither the company nor any other party intends to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
Qualcomm and Snapdragon are trademarks or registered trademarks of Qualcomm Incorporated.
Qualcomm Snapdragon is a product of Qualcomm Technologies, Inc. and/or its subsidiaries.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210708005107/en/
Contacts
Investors
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
U.S. media
Stephanie Simon
+1 617 581 9333
stephanie@tenbridgecommunications.com

















