Provides Tetra with stable supply of high-quality ingredients and regulatory-approved services to satisfy clinical trials and prescription products
Diversifies Akanda into cancer pain medical market as a specialized manufacturer of cannabis-based drugs for use in FDA clinical trials and pharmaceutical markets
Akanda Corp. ("Akanda") (NASDAQ: AKAN) and Tetra Bio-Pharma (“Tetra”) (TSX: TBP) (OTCQB: TBPMF) (FRA: JAM1), today jointly announced that Akanda will supply Tetra with pharmaceutical grade cannabis flower in a microdose cap form, for use in a Storz & Bickel Mighty Medic Vaporizer for global commercialization of Tetra’s QIXLEEFTM and related products. In addition, Akanda will act as a Contract Development and Manufacturing Organization (CDMO) for Tetra’s clinical drug and commercial supply programs. With this project, Akanda becomes a CDMO in addition to being an EU GMP cannabis manufacturer, marking Akanda’s first entry into cannabinoid drug development, which is a new and growing market opportunity for the company, while Tetra secures a stable supply of high-quality ingredients and regulatory-approved services to satisfy clinical trials and commercialization.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220712005519/en/
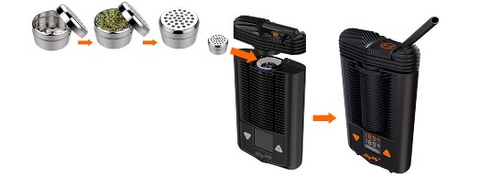
QIXLEEF (TM) product has been designed specifically for use in the Storz & Bickel Mighty Medic Vaporizer, the first medically approved portable cannabis vape, pictured here with dosing capsules filled at Holigen. (Photo: Business Wire)
QIXLEEF™ is a proprietary botanical inhaled investigational new drug currently being studied in two U.S. Food and Drug Administration (FDA) authorized clinical trials: 1) REBORN©1, a Phase 2 study authorized by the FDA to evaluate inhaled cannabinoids against a class of immediate-release oral opioids for the management of breakthrough cancer pain, and 2) PLENITUDE©, a Phase 2 multicenter clinical trial authorized by the FDA to evaluate the safety and efficacy of inhaled cannabinoids to relieve uncontrolled pain in patients with advanced cancer. The companies estimate the total addressable market (TAM) for QIXLEEF™ to be nearly $1.7 billion by 20281.
Under the multi-year agreement, Akanda will supply Tetra with high-quality, premium THC and CBD flower, and will provide regulatory, quality and pharmaceutical manufacturing services for the QIXLEEFTM clinical drug development and marketing authorization from its Portugal operations. The supply of the active pharmaceutical ingredient starts in [the third quarter of] 2022 and is anticipated to increase incrementally over the succeeding years based on growing demand and commercializing of Tetra’s cannabinoid-derived medicines.
Akanda will provide Tetra with a range of services, including regulatory affairs, quality control and stability testing through Akanda’s internal lab, as well as manufacturing capabilities. Upon FDA approval, the anticipated supply commitments could reach [over 10 metric tonnes] per year.
Tetra, a leader in drug discovery and development for cannabinoid-based medicines, is focusing on therapeutic areas of inflammation, pain, ophthalmology and oncology through a robust pipeline using multiple delivery systems.
“This supply agreement with Tetra is a major milestone in Akanda’s journey in becoming a cannabis platform company serving all regulated markets in the EMEA region,” commented Tej Virk, CEO of Akanda. “In supporting a terrific partner with a mission to improve patient health and quality of life though cannabinoid-derived medicine, we are demonstrating that cannabis can fit into the traditional public sector model, with the expectation of reimbursement. Simultaneously, we are productively utilizing our diverse capabilities to support clinical trials for pharma grade cannabinoids. If approved, we expect to provide flower for the authorized compound, potentially creating a significant, incremental revenue stream for Akanda. This opportunity could only have been possible with our state-of-the-art facilities that we gained through the acquisition of Holigen in May.”
“This collaboration transitions our Sintra facility into a global CDMO for cannabinoid-based pharmaceuticals as we build up our internal laboratory capacity and manufacturing under EU GMP,” commented Dr. Akkar-Schenkl, President of Akanda. “Together with Tetra we are aiming to become the ambassadors for cancer pain treatment. The pharmaceutical grade flower and the level of pharmaceutical excellence in manufacturing, quality operations and regulatory affairs we will be providing into these projects is our fundamental commitment to worldwide palliative care in pain treatment in the field of oncology. The bioburden quality of the flower we will be providing for this delicate patient population can only be managed under stringent manufacturing conditions, special regulatory and pharmaceutical know-how.”
“Tetra has been looking for quite a while to find a Global strategic CDMO partner, and we believe that Akanda is a perfect fit from a vision standpoint. This partnership will allow Tetra to secure a robust and trustable source for its clinical drug supply and for QIXLEEF™ commercialization plans. Aside from quality, Akanda will rapidly automate our process and increase our capacity, resulting in a 67% reduction of our cost of goods sold (COGS),” commented Guy Chamberland, M.Sc., Ph.D., Chief Executive Officer and Chief Regulatory Officer at Tetra. “Establishing a defined source of high-quality ingredients is important for Tetra, and we are excited to advance a productive collaboration with Akanda as we advance target drugs through the regulatory process.”
About Akanda Corp.
Akanda (NASDAQ: AKAN) is an international medical cannabis and wellness platform company seeking to help people lead better lives through improved access to high quality and affordable products. Akanda’s portfolio includes Bophelo Bioscience & Wellness, a GACP qualified cultivation campus in the Kingdom of Lesotho in Southern Africa; Holigen, a Portugal-based cultivator, manufacturer and distributor with a prized EU GMP certified indoor grow facility; and CanMart, a UK-based fully licensed pharmaceutical importer and distributor which supplies pharmacies and clinics within the UK. The company’s seed-to-patient supply chain also includes partnerships with Cellen Life Sciences’ Leva Clinic, one of the first fully digital pain clinics in the UK, and Cantourage, which operates a platform for bringing medical cannabis to Europe.
Connect with Akanda: Email | Website | LinkedIn | Twitter | Instagram
About Tetra Bio-Pharma
Tetra Bio-Pharma (TSX: TBP) (OTCQB: TBPMF) (FRA: JAM1) is a leader in cannabinoid-derived drug discovery and development with a FDA and a Health Canada cleared clinical program aimed at bringing novel prescription drugs and treatments to patients and their healthcare providers. Their evidence-based scientific approach has enabled them to develop a pipeline of cannabinoid-based drug products for a range of medical conditions, including pain, inflammation, and oncology. With patients at the core of what they do, Tetra Bio-Pharma is focused on providing rigorous scientific validation and safety data required for inclusion into the existing biopharma industry by regulators, physicians and insurance companies.
Connect with Tetra: Email | Website | LinkedIn | Twitter | Instagram
Forward-looking Statements
Some statements in this release may contain forward-looking information. All statements, other than of historical fact, that address activities, events or developments that Akanda and/or Tetra believes, expects or anticipates will or may occur in the future (including, without limitation, statements regarding potential acquisitions and financings) are forward-looking statements. Forward-looking statements are generally identifiable by use of the words "may", "will", "should", "continue", "expect", "anticipate", "estimate", "believe", "intend", "plan" or "project" or the negative of these words or other variations on these words or comparable terminology. Forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond the ability of either company to control or predict, that may cause the actual results of the companies to differ materially from those discussed in the forward-looking statements. Factors that could cause actual results or events to differ materially from current expectations include, among other things, without limitation, the inability of the companies to obtain sufficient financing to execute their respective business plans; competition; regulation and anticipated and unanticipated costs and delays, the success of the companies’ research and development strategies, including the success of this product or any other product, the applicability of the discoveries made therein, the successful and timely completion and uncertainties related to the regulatory process, the timing of clinical trials, the timing and outcomes of regulatory or intellectual property decisions and other risks disclosed in the Akanda’s and Tetra's public disclosure record on file with the relevant securities regulatory authorities. Although Akanda and Tetra have attempted to identify important factors that could cause actual results or events to differ materially from those described in forward-looking statements, there may be other factors that cause results or events not to be as anticipated, estimated or intended. Readers should not place undue reliance on forward-looking statements. The forward-looking statements included in this news release are made as of the date of this news release, and Akanda and Tetra do not undertake an obligation to publicly update such forward-looking statements to reflect new information, subsequent events or otherwise unless required by applicable securities legislation.
Neither the TSX Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Exchange) accepts responsibility for the adequacy or accuracy of this release.
1 Source: Derived from DelveInsight data encompassing the seven major markets, or 7MM, including the United States, Germany, France, Italy, Spain, United Kingdom and Japan.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220712005519/en/
Contacts
Akanda Investor Contact
Matt Chesler, CFA
FNK IR
1 (646) 809-2183
ir@akandacorp.com
Tetra Investor and Media Contact
Ms. Natalie Leroux – Director
1 (833) 977-7575
investors@tetrabiopharma.com
media@tetrabiopharma.com
Akanda U.S. Media Contact
Annie Grant
Allison + Partners
akanda@allisonpr.com
Akanda Europe Media Contact
Imogen Saunders
Irvine Partners
imogen@irvinepartners.co.uk

















