
Altimmune Inc. (NASDAQ: ALT) is a clinical-stage biotechnology company specializing in treating liver disease and obesity. Their lead drug, Pemvidutide, has garnered much attention as a GLP-1/glucagon dual agonist similar to the weight-loss treatments Mounjaro and Zepbound by Eli Lilly and Co. (NYSE: LLY). The Semaglutide revolution has triggered a GLP-1 gold rush in the medical sector driven by the success of Ozempic and Wegovy, produced by Novo Nordisk A/S (NYSE: NVO). The market is eagerly seeking new GLP-1 obesity treatments and bidding up promising candidate stocks, as evidenced by the 310% year-to-date (YTD) performance for Viking Therapeutics Inc. (NASDAQ: VKTX) shares thanks to its VK2735 lead GLP-1/glucagon drug.
Pemvidutide's Double Threat
Altimmune's lead candidate, Pemvidutide, addresses both obesity and liver disease as they two can be linked. Non-alcoholic steatohepatitis (NASH) is fatty liver that's not caused by alcohol. When fat builds up in the liver, it can lead to fibrosis (buildup of scar tissue) and cirrhosis (hardening of the liver), which can lead to liver cancer. Nearly 70% to 80% of NASH patients suffer from obesity. A third disorder that Pemvidutide could address is metabolic dysfunction-associated steatohepatitis (MASH), which is addressed in its Phase 2b IMPACT study. The FDA granted a fast-track designation to MASH. Study results are expected in Q1 2025.
How Pemvidutide Works
Pemvidutide is a novel and investigational peptide that acts on two hormones. The glucagon-like peptide (GLP-1) is a receptor that stimulates insulin secretion while suppressing glucagon secretion. Insulin helps lower blood sugars by promoting glucose uptake by cells, while glucagon reacts oppositely by increasing sugar levels. Glucagon promotes fat burning in the tissues, including the liver. GLP-1 suppresses appetite while inducing the feeling of being full, while insulin production regulates blood sugar levels, which can improve metabolic health. Increasing glucagon receptor activity promotes fat burning, leading to a reduction of fat in the liver.
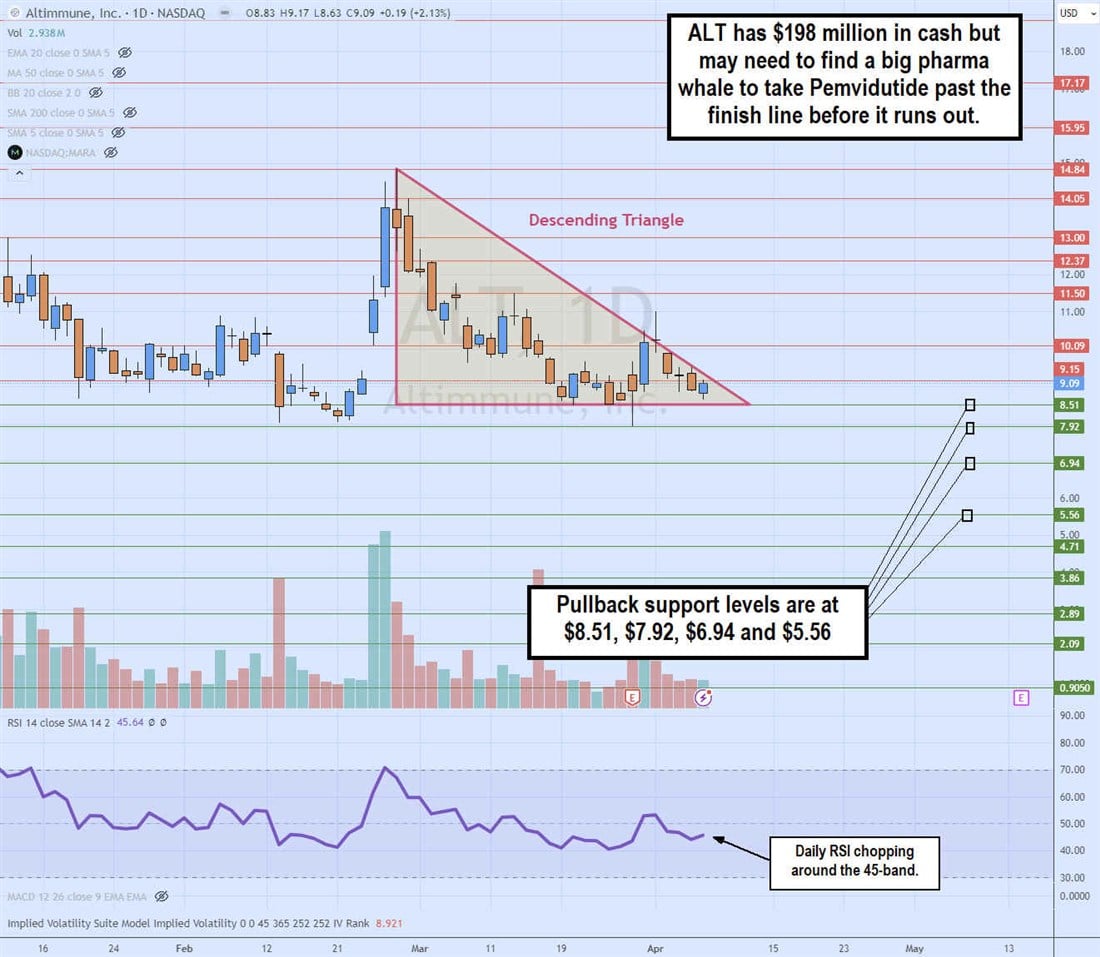
Daily Descending Triangle
The daily candlestick chart on ALT illustrates a descending triangle pattern. The descending trendline formed at the $14.84 swing high on February 28, 2024, capping bounces at lower highs. The flat-bottom lower trendline is at $8.51, meeting at the apex point. ALT will soon break out through the upper trendline or break down through the flat-bottom lower trendline. The daily relative strength index (RSI) is chopping sideways at the 45-band. Pullback support levels are at $8.51, $7.92, $6.94 and $5.56.
Good News: Weight Loss, Fat Reduction and Lean Mass Preservation
Altimmune's initial top-line results from its MOMENTUM phase 2 clinical trials were considered successful. Weight loss averaged 15.6% at the highest dose and 10.3% at the 1.2mg lowest dosing. Nearly 48% of patients in the 48-week trial who were initially categorized as having obesity, a body mass index of 30 or greater, were no longer qualified as obese at the end of the study.
Additionally, lean mass preservation was also prevalent, as 25.5% of weight loss came from lean mass loss compared to 39% lean mass loss with GLP-1 drugs like Ozempic. Pemvidutide illustrated significant loss of the liver without heart rate increases. Up to 78.6% of patients with excess liver fat normalized their fat content.
Bad News: Tolerability and Dropout Rates
One of the biggest drawbacks has been the gastrointestinal distress caused by a GLP-1/glucagon dual agonist. GLP-1 is known to cause gastrointestinal problems, but glucagon stimulation can exacerbate the problem. The tolerability has been an issue with Pemvidutide as the discontinuation rate rose with higher doses. Adverse effects (AEs) are unfavorable and unintended symptoms that can range from mild nausea to severe allergic reactions to organ failure.
Serious adverse effects (SAEs) are those that are potentially life-threatening, require inpatient hospitalization or cause significant disability. At the highest dose of 2.4 mg weekly, drug-related AE discontinuations rose to 15.5%, up from 2.1% placebo or 6.6% for Wegovy. Nearly 50% of participants experienced nausea, 27.8% vomiting and 18.6% diarrhea at the highest dosing.
Cash Crunch and Dilution
Altimmune reported total cash and cash equivalents of $198 million at the end of December 31, 2023. This was after net losses of $31.6 million in the quarter. Altimmune has an at-the-market (ATM) authorization in place to still sell up to $135.7 million in stock.
Holding Out for a Hero
The hope is that a big pharma whale will soon come on board as a partner to offset research costs and partner for a potential launch if it gets FDA approval. Altimmune has had a history of failing to launch or coming up short, like its adCOVID vaccine, which flunked its Phase 1 clinical trials by failing to produce an adequate immune response in 2021.
Altimmune analyst ratings and price targets are at MarketBeat. Altimmune peers and competitor stocks can be found with the MarketBeat stock screener. Altimmune has a 17.65% short interest.

















