FAYETTEVILLE, Ark. - March 23, 2022 - PRLog -- Lineus Medical announces the publication of a pivotal clinical study performed at Hartford Hospital, part of Hartford HealthCare in Hartford, Connecticut, in the March edition of The Journal of Infusion Nursing. The study was a prospective randomized clinical study that evaluated the safety and clinical effectiveness of SafeBreak Vascular, the first FDA cleared Force-Activated Separation Device.
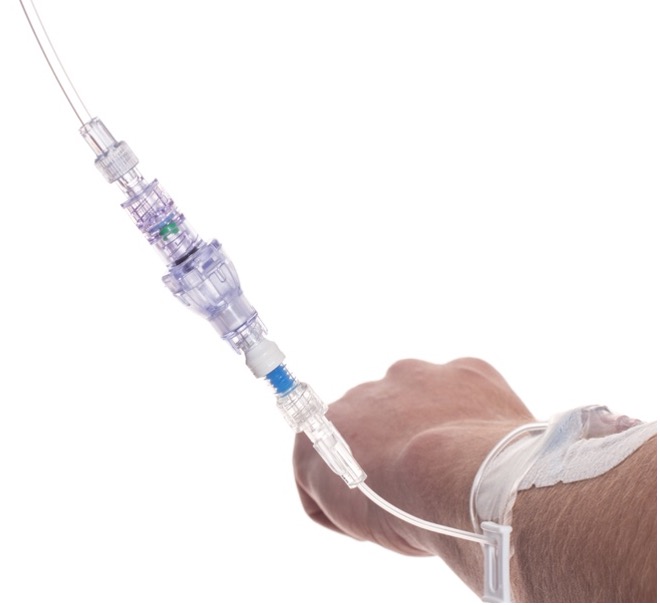
SafeBreak Vascular is a break-away connector that fits into any peripheral IV line in the world. The device is designed to separate when damaging force is placed on an IV line, reducing vein irritation and the chances of the IV dislodging. When the device separates, valves on each side of the device close to stop the flow of medication from the pump and blood flow from the patient's IV catheter. The separated SafeBreak can be replaced with a new, sterile SafeBreak and the IV restarted without the need for another needlestick for the patient.
SafeBreak Vascular reduces the failure of peripheral IV lines and the negative cascade of events that occurs when an IV restart is required. The clinical literature shows that over 235 million peripheral IV lines are placed each year in the US and that these IV lines fail before their intended end of use a staggering 46% of the time on average. The cost of an IV restart is approximately $64.84, and IV restarts are estimated to cost the US healthcare system over $7 billion annually.1-4 The 302-patient study showed that the use of SafeBreak Vascular on peripheral IV catheters resulted in a 46% reduction of complications requiring an IV restart in the SafeBreak Vascular group when compared to the control group5.
"We are thankful for Lee Steere, Dr. Gregory Panza, Dr. Adam Steinberg, and the rest of the research team at Hartford Hospital who helped support this clinical study during the heart of the COVID-19 pandemic." Vance Clement, CEO of Lineus Medical stated. "SafeBreak Vascular was shown to significantly reduce IV mechanical complications requiring IV restart, eliminate spills and save nurses a great deal of time."
"Completion of this study was critical to the 2021 FDA De Novo clearance of SafeBreak Vascular and we're excited to share these fantastic results with the world.", said Lineus Medical's founder and Chief Technology Officer, Spencer Jones.
1Helm, R.E., et al., Accepted but Unacceptable: Peripheral IV Catheter Failure. Journal of Infusion Nursing. 2015;38(3):189-203
2Costantino TG, et al., Ultrasonography-guided PIV access versus traditional approaches. Ann Emerg Med. 2005;46,456–461.
3iData Research Inc Report: US Market on Vascular Access Devices and Accessories, Vancouver, Canada. 2016:p.342.
4Jones RK. Short peripheral catheter quality and economics: the intravenous quotient. J Infus Nurs. 2018;41(6):365-371.
5Data on file.
MKG-0132 Rev00
Photos: (Click photo to enlarge)![]()



![]()
Read Full Story - Results of Pivotal SafeBreak® Vascular Clinical Study Published in Journal of Infusion Nursing | More news from this source
Press release distribution by PRLog
Results of Pivotal SafeBreak® Vascular Clinical Study Published in Journal of Infusion Nursing
March 23, 2022 at 13:46 PM EDT












