Liqomics is proud to announce the launch of LymphoVista
LymphoVista (https://liqomics.com/detect-lymphoma-lymphovista/) is an advanced ctDNA-based assay specifically designed for minimal residual disease (MRD) monitoring and genotyping in lymphoma patients. LymphoVista leverages high-sensitivity circulating tumor DNA (ctDNA) detection, setting a new standard in MRD testing across lymphomas including Hodgkin Lymphoma, Diffuse Large B-Cell Lymphoma (DLBCL), Central Nervous System (CNS) Lymphomas and Follicular Lymphoma.
Revolutionizing MRD Testing in Hodgkin Lymphoma and Other Lymphomas
LymphoVista addresses a critical need in genotyping and monitoring of MRD in lymphoma, providing a level of sensitivity, specificity, and accuracy that has been lacking in many current MRD assays. "Many existing cfDNA assays fail to meet the precision needed for lymphoma-specific MRD monitoring," said Sven Borchmann MD/PhD, founder and managing director of Liqomics. "With LymphoVista, we introduce a validated assay that can monitor MRD with accuracy as low as a few molecules of ctDNA per million enabling clinicians to make more data-driven treatment decisions."
LymphoVista HL Validation: High Sensitivity and Specificity
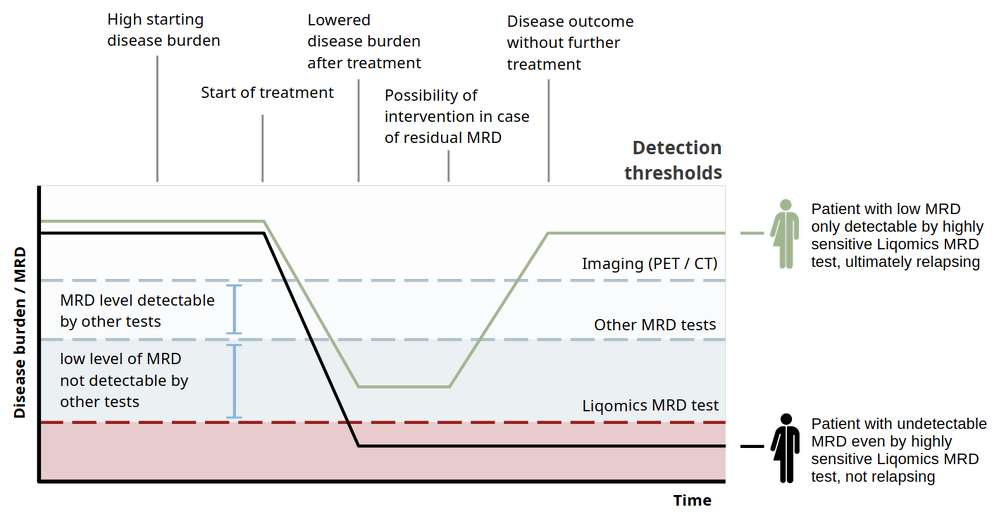
Validation studies confirm LymphoVista as a high-precision tool for MRD assessment. Key validation metrics include:
- Sensitivity > 91% and Specificity > 99.99% for variant detection (genotyping) allowing reliable identification of SNPs and InDels
- Limit of Detection (LoD) for MRD: 0.000654%, making it highly suitable for detecting minimal disease presence.
Clinical Validation Demonstrates Prognostic Power in Hodgkin Lymphoma
A clinical validation study for LymphoVista was performed in Hodgkin lymphoma (HL) involving 72 patients. This study demonstrated that MRD detection following two cycles of therapy effectively stratified patients based on risk. Patients MRD-negative patients after two cycles of chemotherapy had a 4-year progression-free survival (PFS) of 95.3% (95%-CI: 88.7 − 100) while patients who were MRD-positive after two cycles of chemotherapy had a 4-year PFS of 72.2% (95%-CI: 41.5-100) resulting in a hazard ratio of 6.9 (95%-CI: 4.5-10.6). In conclusion, MRD-negative patients showed very good outcomes, while MRD-positive patients had a significantly higher risk of relapse. This clinical performance study shows LymphoVista is a valuable tool for MRD detection in lymphoma, particularly Hodgkin lymphoma.
LymphoVista HL: Setting a New Benchmark for MRD-Guided Clinical Trials in Lymphoma
The launch of LymphoVista heralds a new era in personalized medicine for lymphoma, laying a foundation for MRD-guided clinical trials. The assay’s validated accuracy and sensitivity support its use in clinical trials and can inform treatment decisions based on real-time MRD results.
For more information on LymphoVista or collaboration opportunities with Liqomics, please contact: https://liqomics.com/contact/
About Liqomics
Liqomics is a pioneering molecular diagnostics company focused on developing cutting-edge circulating tumor DNA (ctDNA) and cell-free DNA (cfDNA) assays to advance personalized medicine in oncology, with a strong emphasis on minimal residual disease (MRD) detection and genotyping in lymphomas and other hematological malignancies. Liqomics uses state-of-the-art technology for its assays which are rigorously validated to create highly sensitive diagnostic tools that enable precise disease monitoring and optimized treatment decisions.
The company is headquartered in Cologne, Germany. Liqomics' mission is to empower clinicians and patients with data-driven insights through validated cfDNA-based assays, ensuring that patients receive timely and effective care. Through its dedication to innovation and precision, Liqomics aims to set new standards in MRD monitoring and genotyping, enabling clinicians to make real-time adjustments in treatment plans based on the genetic profile and MRD status of each patient.
Media Contact
Company Name: Liqomics GmbH
Contact Person: Sven Borchmann MD/PhD Managing Director
Email: Send Email
Phone: (+49) 221 69 056 597
Address:Hermulheimer Str. 68
City: Koln 50969
Country: Germany
Website: https://liqomics.com/













