
Geron Inc. (NASDAQ: GERN) is a clinical-stage biotech in the medical sector focused on developing cell therapies to treat certain types of blood cancers and myeloid hematologic malignancies. Their lead drug is imetelstat, which blocks a protein called telomerase to treat specific blood cancers like myelofibrosis and myelodysplastic syndromes. Its shares surged 92% after a Federal Drug Administration (FDA) advisory committee voted 12 to 2 in favor of an FDA approval.
How is Cell Therapy Different From Gene Therapy
Gene therapy has been getting a lot of headlines in 2024, notably for CRISPR Therapeutics AG (NASDAQ: CRSP) and Vertex Pharmaceuticals Inc. (NASDAQ: VRTX) FDA approval for its CRISPR/cas-9 NASDAQ: CRSP">gene-editing treatment for sickle cell disease (SCD). Gene therapy involves modifying the genetic materials in a cell to prevent or treat a hereditary disease. Cell therapy involves using whole cells that are introduced into the body to repair or replace damaged tissue and help the immune system fight off diseases like cancer. Both methods seek to treat disease at a cellular level but involve different mechanisms to achieve this.
Imetelstat Applications
Imetelstat can be used as a late-line therapy for transfusion-dependent anemia, which occurs in a blood disorder called myelodysplastic syndrome (MDS). Imetelstat would be used in cases where other therapies have been unsuccessful. In MDS, the bone marrow can't produce enough healthy blood cells. Therefore, abnormal blood cells overcrowd healthy blood cells, which can lead to anemia and fatigue. People suffering from this condition require regular blood transfusions to help manage symptoms.
Imetelstat was developed to treat transfusion-dependent anemia in adult patients with lower-risk MDS. It's a telomerase inhibitor, which blocks the activity of an enzyme called telomerase. Telomerase enables cancer cells to maintain their ability to divide indefinitely. Limiting telomerase can help slow down or stop tumor growth by preventing them from duplicating. Check out the sector heatmap on MarketBeat.
First Line Treatments
First-line treatment in the anemia therapy segment can be performed using Reblozyl, which was FDA-approved in 2023. It was developed under a collaboration between Bristol Myers-Squibb Co. (NYSE: BMY) and Merck & Co. (NYSE: MRK). The FDA has a target action date of June 16, 2024, for its Prescription Drug User Fee Act (PDUFA). The FDA advisory panel vote is non-binding, but the FDA usually sides with the advisory panel for approvals when deciding on approval.
Staying Afloat Until Final FDA Approval
On February 28, 2024, Geron reported an EPS loss of 9 cents, which was a penny beat against the consensus analyst estimates. Revenues fell 77% YoY to $0.02 million versus $0.06 million analyst estimates. The company lost $194 million in Q1 2024. Total operating expenses for 2024 are expected to range from $270 million to $280 million. The company had $378.1 million in cash and cash equivalents. Geron has been raising cash through 2023 through secondary offerings of its common shares. Management expects to hire more workers, up from 114 to 270, by the end of the calendar year.
Comments from the CEO
Geron, Executive Vice President and Chief Medical Officer Fay Feller, MD, commented, "We are pleased with the Committee's decision to recognize the positive clinical benefit/risk profile of imetelstat for the treatment of transfusion-dependent anemia in adult patients with lower-risk MDS. There are few treatment options and significant unmet medical need remains for these patients, particularly among those with difficult-to-treat subtypes of this blood cancer."
Feller continued, "We believe that imetelstat has the potential to be an important new medicine for patients and look forward to continuing our collaboration with the FDA as they complete their review of our New Drug Application."
Needham Raises Price Target
Needham already had a buy rating on GERN. After the successful FDA Advisory Panel vote, Needham raised its price target to $5, up from $4.
Geron analyst ratings and price targets are at MarketBeat. Geron's peers and competitor stocks can be found with the MarketBeat stock screener.
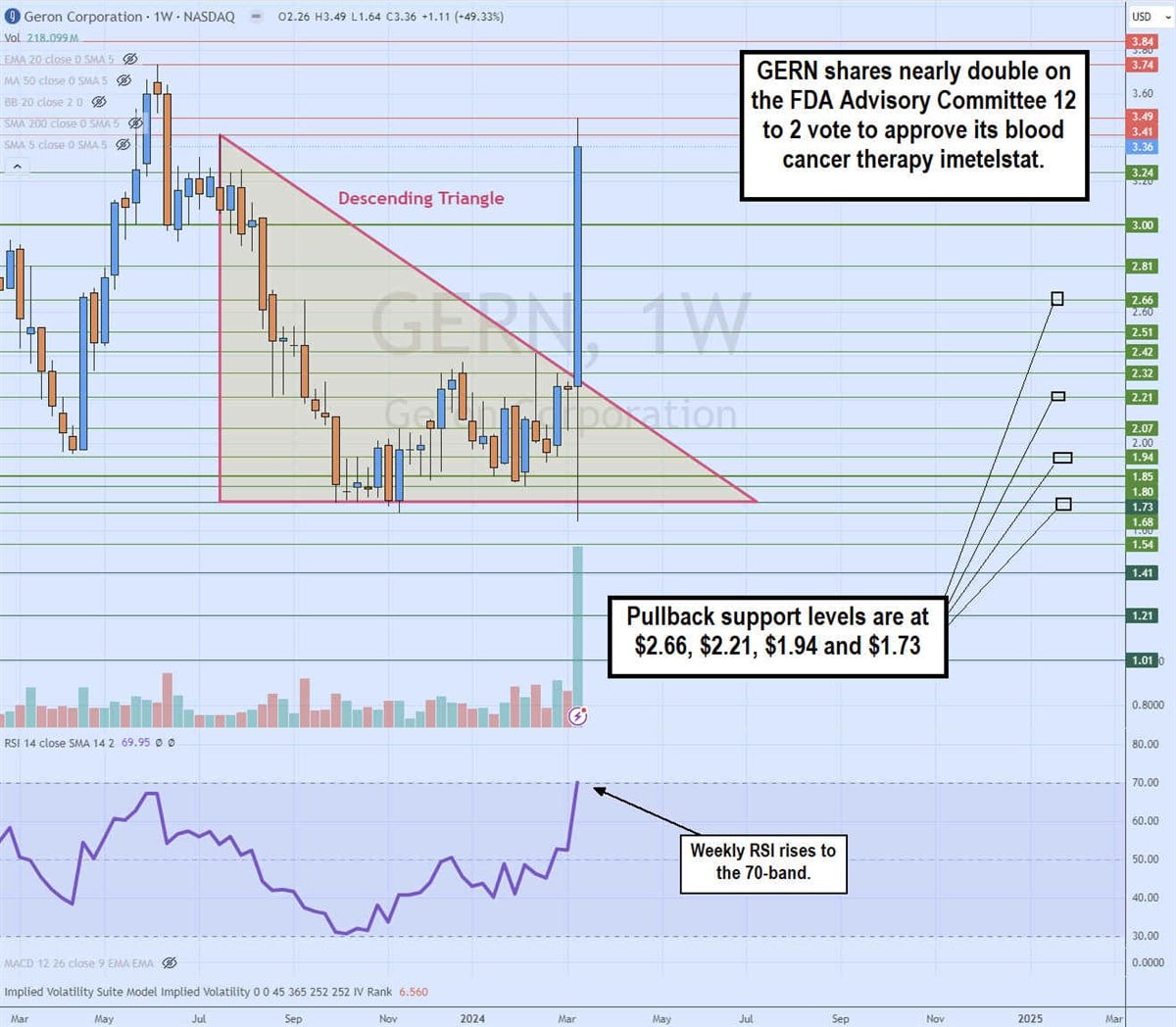
Weekly Descending Triangle Breakout
The daily candlestick chart for GERN illustrates a daily descending triangle breakout pattern. The descending trendline formed at the $3.41 peak on July 17, 2023. GERN shares collapsed to the flat-bottom trendline support at $1.73. The bounce attempts off that area were capped at the descending trendline. The FDA Advisory Panel is a scheduled event and GERN shares sold off ahead of the decision. Upon receiving the panel decision, GERN shares spiked to a high of $3.49 before settling back down. The weekly relative strength index (RSI) surged to test the 70-band. Pullback support levels are at $2.66, $2.21, $1.94 and $1.73.
















